- Registration Service of Medical Devices
- CRC services Medical Device Business License Medical Device Registration Contract Research Organization ISO 13485 Quality System Certification GMP CE Certification Service FDA Registration (Filing) Software Integrity Verification Service Production process validation service ISO 15378 Medical Package System Certification ISO 13485 Internal Auditors Training Regulatory Services For Medical Devices
- Information security service of medical
- ISO 27001 Information Security Certification ISO 20000 Information Service Certification Information system grade protection filing Business Continuity Management Services Internal Auditor Training
- Medical Software Development
- Software of Good Supply Practice(GSP) Production System Development Customized software development
- CONTACT US
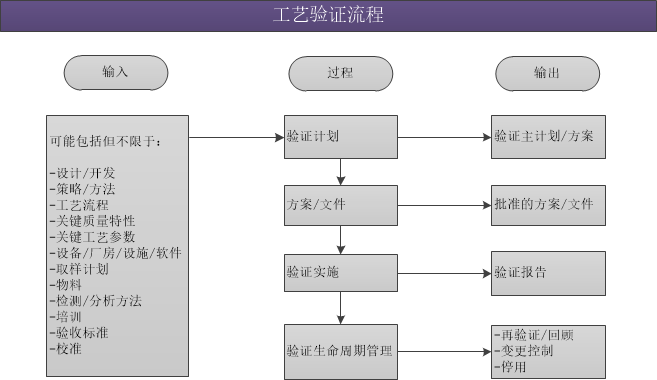
工藝驗證:從工藝設計階段到商業生產的整個過程中,對數據進行收集和評價,建立工藝能始終如一地交付出優質產品中的科學證據。工藝驗證涉及整個產品生命周期和生產中發生的一系列活動。包括3階段:
第一階段 - 工藝設計:在開發和放大活動過程中獲得的知識基礎上,在此階段對商業化生產工藝進行定義。
第二階段 - 工藝確認:在此階段,對工藝設計進行評估,以確認工藝是否具備可重現的商品化制造能力。
第三階段 - 持續工藝確認:在日常生產中獲得工藝處于受控狀態的持續的保證。
NEXT: NOTHING
